Gaseous iodine pentafluoride, IF5, can be prepared by the reaction of solid iodine and gaseous fluorine: I2 (s)+5F2 (g)?2IF5 (g) A 4.90?L flask containing 9.80g I2 is charged with 9.80g F2, and the reaction proceeds until one of the reagents is completely consumed.
WS 7.5 Partial Pressures (Dalton’s Law)
1 answer 1 watching 680 views amaranthhorse137 Lv1 18 Apr 2020 Gaseous iodine pentafluoride, IF 5, can be prepared by the reaction of solid iodine and gaseous fluorine: I 2 ( s) + 5 F 2 ( g) 2 IF 5 ( g) A 5.00-L flask containing 10.0 g of I 2 is charged with 10.0 g of F 2, and the reaction proceeds until one of the reagents is completely consumed.

Source Image: pearson.com
Download Image
Sep 17, 2023After the reaction is complete, the temperature in the flask is 125∘C 125 ∘ C. (a) What is the partial pressure of IF5 I F 5 in the flask? (b) What is the mole fraction of IF5 I F 5 in the flask? Gaseous iodine pentafluoride, IF5 I F 5, can be prepared by the reaction of solid iodine and gaseous fluorine:
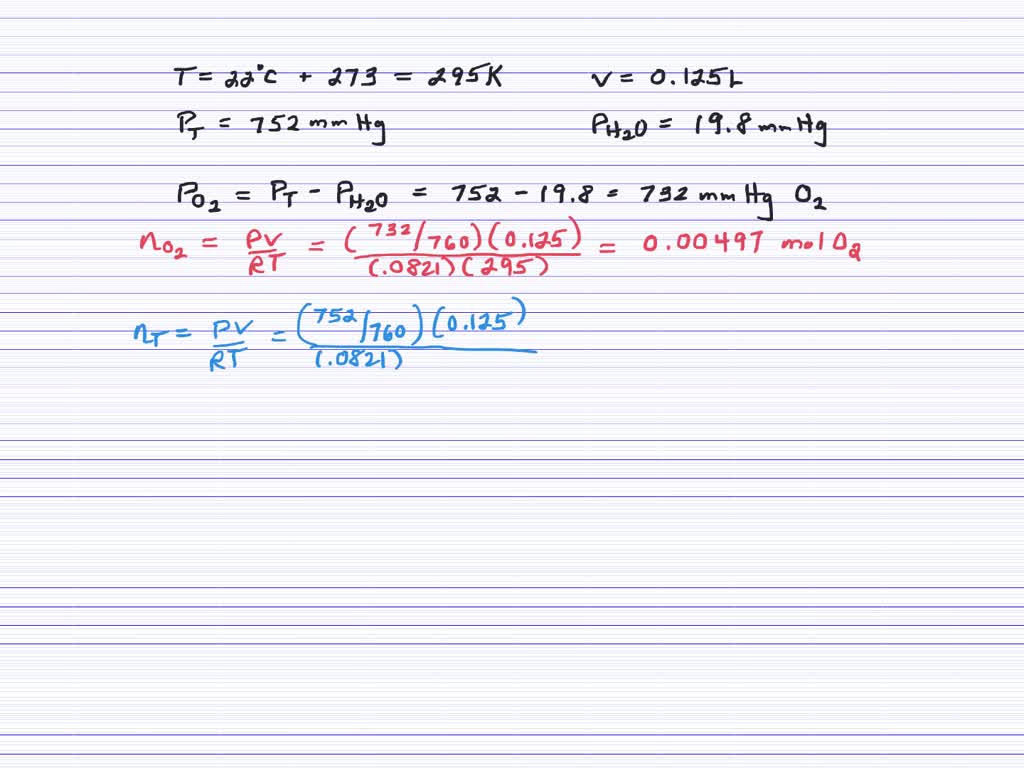
Source Image: numerade.com
Download Image
Partial Pressure Video Tutorial & Practice | Channels for Pearson+ Partial pressures are determined by the mole fraction of the gas in the mixture, thus there are no specific values for gases. For example, if a mixture contains 1 mole of gas A and 2 moles of gas B and the total pressure is 3 atm. The partial pressure of gas B is 2 atm and 1 atm for gas A. 🙂 1 comment. ( 3 votes)

Source Image: pearson.com
Download Image
What Is The Partial Pressure Of If5 In The Flask
Partial pressures are determined by the mole fraction of the gas in the mixture, thus there are no specific values for gases. For example, if a mixture contains 1 mole of gas A and 2 moles of gas B and the total pressure is 3 atm. The partial pressure of gas B is 2 atm and 1 atm for gas A. 🙂 1 comment. ( 3 votes) What is the partial pressure of IF_5 I F 5 in the flask? Solutions Verified Solution A
Gaseous iodine pentafluoride, IF5, can be prepared by the reacti… | Channels for Pearson+
I2 (s)+5F2 (g)→2IF5 (g) A 5.00 −L flask containing 10.0 g I2 is charged with 10.0 g F2, and the reaction proceeds until one of the reagents is completely consumed. After the reaction is complete, the temperature in the flask is 125 ∘C. I’m completely stuck on this. I get .85 atm but apparently that isn’t This problem has been solved! ⏩SOLVED:Gaseous iodine pentafluoride, IF5, can be prepared by the… | Numerade
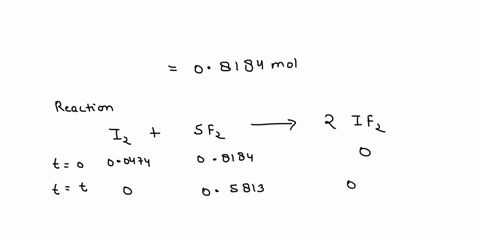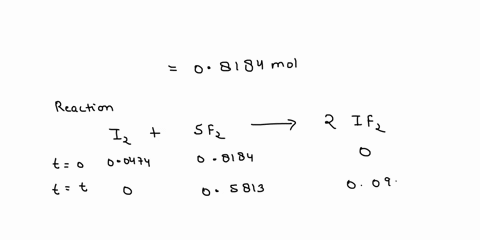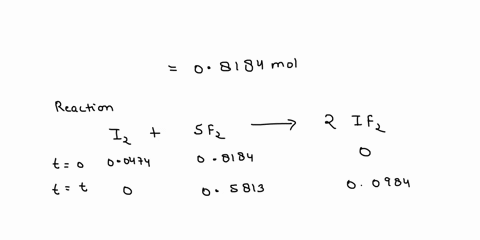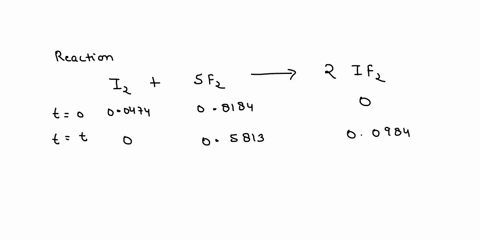
Source Image: numerade.com
Download Image
Partial Pressures (Example) – YouTube I2 (s)+5F2 (g)→2IF5 (g) A 5.00 −L flask containing 10.0 g I2 is charged with 10.0 g F2, and the reaction proceeds until one of the reagents is completely consumed. After the reaction is complete, the temperature in the flask is 125 ∘C. I’m completely stuck on this. I get .85 atm but apparently that isn’t This problem has been solved!

Source Image: youtube.com
Download Image
WS 7.5 Partial Pressures (Dalton’s Law) Gaseous iodine pentafluoride, IF5, can be prepared by the reaction of solid iodine and gaseous fluorine: I2 (s)+5F2 (g)?2IF5 (g) A 4.90?L flask containing 9.80g I2 is charged with 9.80g F2, and the reaction proceeds until one of the reagents is completely consumed.

Source Image: yumpu.com
Download Image
Partial Pressure Video Tutorial & Practice | Channels for Pearson+ Sep 17, 2023After the reaction is complete, the temperature in the flask is 125∘C 125 ∘ C. (a) What is the partial pressure of IF5 I F 5 in the flask? (b) What is the mole fraction of IF5 I F 5 in the flask? Gaseous iodine pentafluoride, IF5 I F 5, can be prepared by the reaction of solid iodine and gaseous fluorine:
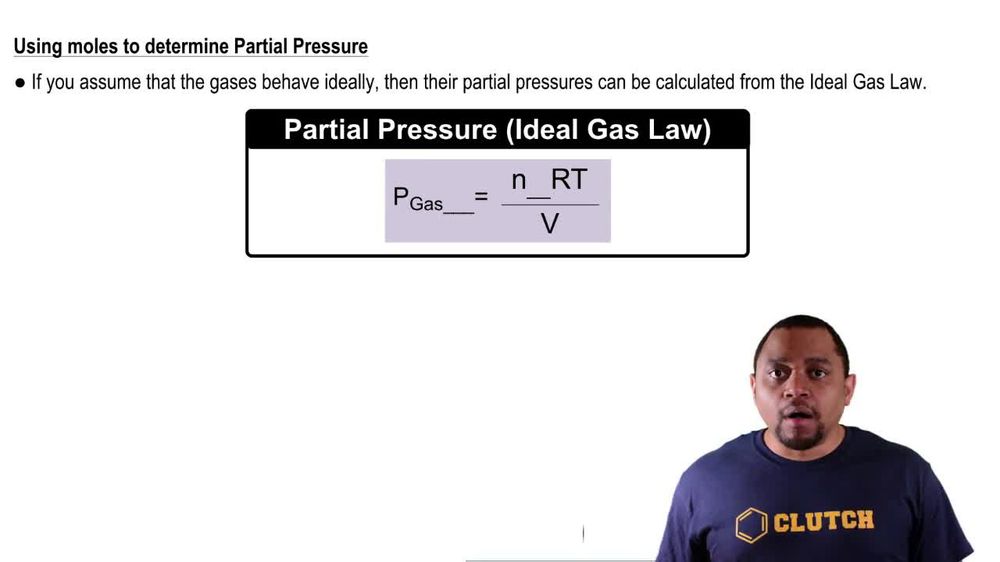
Source Image: pearson.com
Download Image
SOLVED: Iodine and fluorine gases react to form iodine pentafluoride gas(es). I2(g) + 5 F2(g) 2 IF5(g) At a certain temperature and pressure 17.2 L of I2 reacts with 86 L of Dalton’s law of partial pressures states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the component gases: P Total = P gas 1 + P gas 2 + P gas 3 … Dalton’s law can also be expressed using the mole fraction of a gas, x : P gas 1 = x 1 P Total Introduction

Source Image: numerade.com
Download Image
Chlorine gas reacts with fluorine gas to form chlorine trifluorid… | Channels for Pearson+ Partial pressures are determined by the mole fraction of the gas in the mixture, thus there are no specific values for gases. For example, if a mixture contains 1 mole of gas A and 2 moles of gas B and the total pressure is 3 atm. The partial pressure of gas B is 2 atm and 1 atm for gas A. 🙂 1 comment. ( 3 votes)

Source Image: pearson.com
Download Image
Solved Consider the flasks in the following diagram. 2.50 L | Chegg.com What is the partial pressure of IF_5 I F 5 in the flask? Solutions Verified Solution A
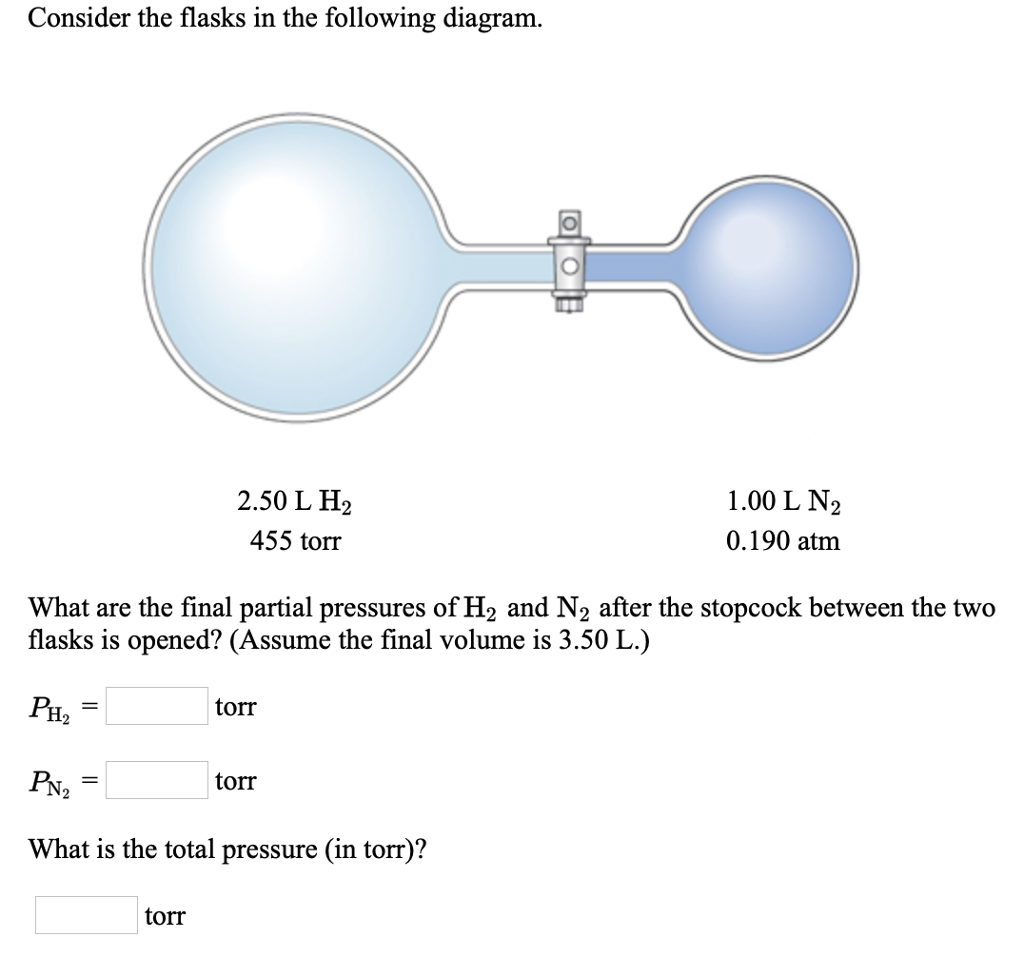
Source Image: chegg.com
Download Image
Partial Pressures (Example) – YouTube
Solved Consider the flasks in the following diagram. 2.50 L | Chegg.com 1 answer 1 watching 680 views amaranthhorse137 Lv1 18 Apr 2020 Gaseous iodine pentafluoride, IF 5, can be prepared by the reaction of solid iodine and gaseous fluorine: I 2 ( s) + 5 F 2 ( g) 2 IF 5 ( g) A 5.00-L flask containing 10.0 g of I 2 is charged with 10.0 g of F 2, and the reaction proceeds until one of the reagents is completely consumed.
Partial Pressure Video Tutorial & Practice | Channels for Pearson+ Chlorine gas reacts with fluorine gas to form chlorine trifluorid… | Channels for Pearson+ Dalton’s law of partial pressures states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the component gases: P Total = P gas 1 + P gas 2 + P gas 3 … Dalton’s law can also be expressed using the mole fraction of a gas, x : P gas 1 = x 1 P Total Introduction